

Now, we can plug all the data into our Theoretical Yield Calculator: Limiting Reactant (O 2) Stoichiometry = 5, Molecular Weight = 31.9988 g/mol, Reactant Mass = 50.00 g Desired Product (H 2O) Stoichiometry = 2, Molecular Weight = 18.01528. In other words, we have an excessive number of acetylene molecules and, accordingly, the limiting reagent will be oxygen. On the other hand, from the reaction equation, we see that for 2 molecules of C 2H 2 there are 5 molecules of O 2. Molecular weights of C 2H 2 and O 2 are 26.03728 g/mol and 31.9988 g/mol respectively. Then the values found must be compared with the stoichiometric coefficients of the chemical reaction in question.
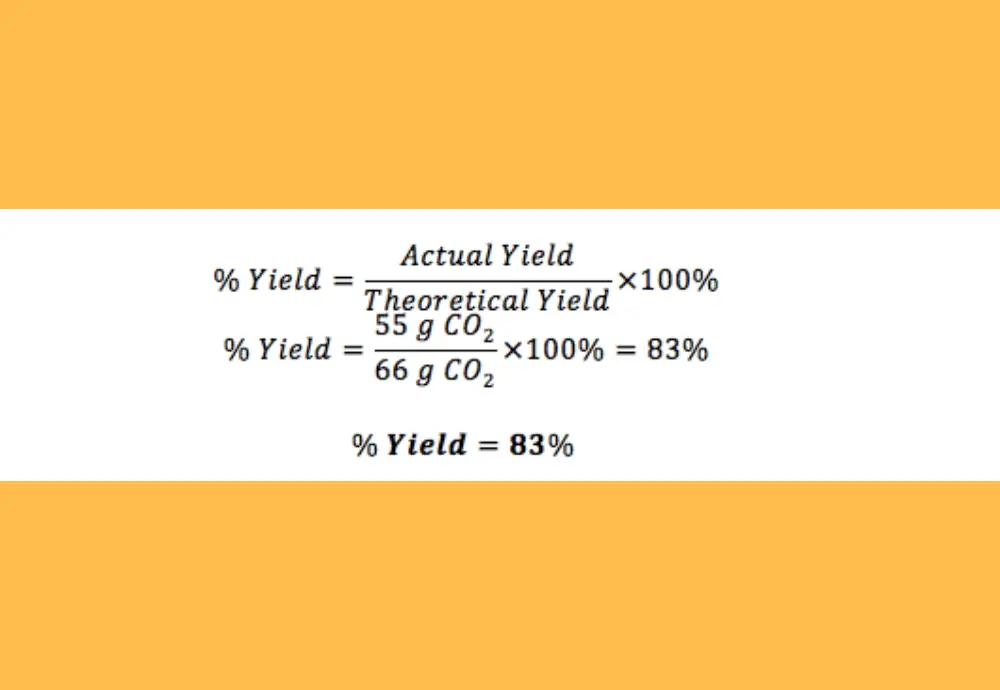
To find the limiting reactant, it is necessary to find out how many moles of the initial reactants are involved in the reaction. The limiting reactant is the one that is in short supply, so that the reaction cannot proceed when it is used up. For this we need to find the limiting reactant in the balanced chemical equation. Then, we have to calculate the theoretical yield. What is the percent yield of water in this reaction?įirst write the following balanced chemical equation: Example of Calculating Percent YieldĬonsider the reaction of 36.00 g of acetylene (C 2H 2) and 50.00 g of oxygen, which produces 10.00 g of water. Percent yield indicates the efficacy of a chemical reaction and is considered in various experiments, such as the simple decomposition of a compound, as a measure of the accuracy of the experiment. Exceeding can also be the result of an error due to incomplete removal of solvent or other impurities from the sample of the reaction product. This can happen if there were other reactions that also formed the product. This means that more of the reaction product was recovered from the reaction than expected. However, there are situations when the percent yield can exceed 100%. This is due to incomplete or competing reactions as well as loss of the reaction product during recovery. Since the actual yield is usually less than the theoretical value, the percent yield is often below 100%. The units of actual and theoretical yield should be the same (moles or grams). Theoretical yield is the amount of reaction product calculated using the stoichiometry of a balanced chemical reaction equation, taking into account the limiting reactant.Actual yield is the amount of a product obtained from a chemical reaction,.Percent yield = (Actual yield / Theoretical yield) * 100% In other words, it is calculated as the actual yield divided by the theoretical yield and multiplied by 100%: Please contact the Office of Communications and Public Relations, Division of Research for further information: or (979) 845-8069.The percent yield, also referred to as percentage yield, is the percent ratio of actual yield to the theoretical yield of a chemical reaction.
Use of the images for non-university purposes is subject to approval. Texas A&M University may or may not have model releases for people photographed on campus, in classrooms, research laboratories, or other areas related to Texas A&M. Hays at 97 or by Jean Wulfson, Division of Research and Graduate Studies, Texas A&M University His primary interests center on defining the molecular mechanisms for enviromental stress (heat and drought) induced grain abortion and impaired starch and protein reserve deposition while also breeding improved heat and drought tolerance during plant grain set and development into new cultivars adopted to the Southern Great Plains.įor more information on this project, contact Dirk B. Hays, assistant professor in the Department of Soil and Crop Sciences, College of Agriculture and Life Sciences, focuses on the genetic and molecular regulation of cereal grain development in wheat and sorghum. This project has identified sources of heat tolerance from Australian and Middle Eastern wheat lines that have significantly improved heat tolerance. Currently grown Texas Wheat cultivars lose 50 percent of their yield potential on a yearly basis due to heat stress during flowering and early seed development. This wheat spike is part of a research project that focuses on breeding improved heat and drought tolerance during the flowering and seed development stages of wheat.


 0 kommentar(er)
0 kommentar(er)
